Summary
We can summarize all the above contents, the type of distortion and the strength of distortion for different type of complexes in the below table:
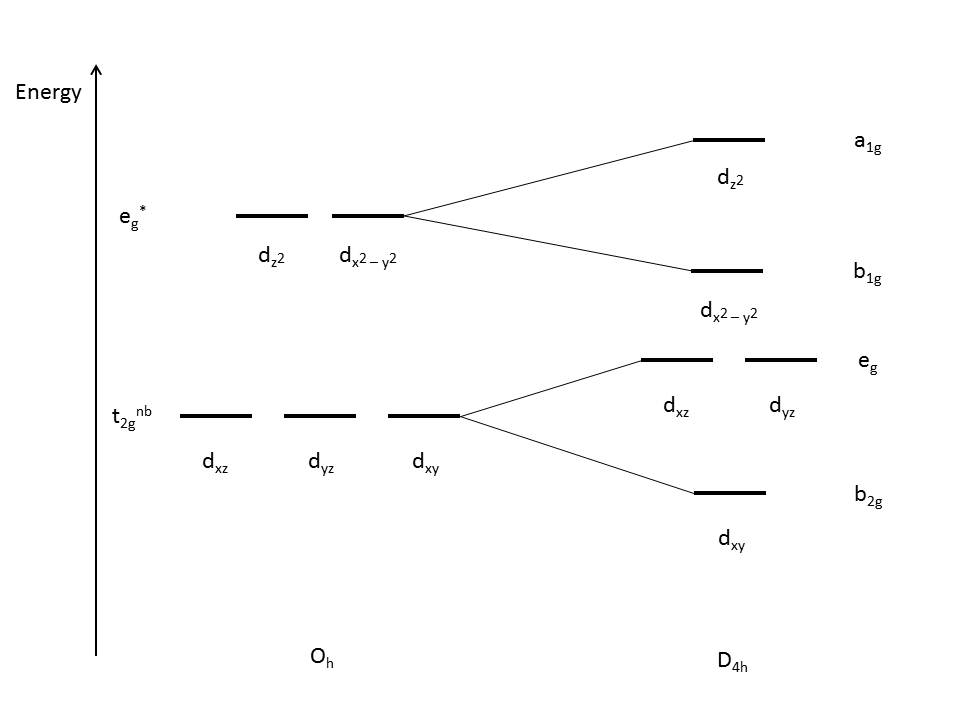
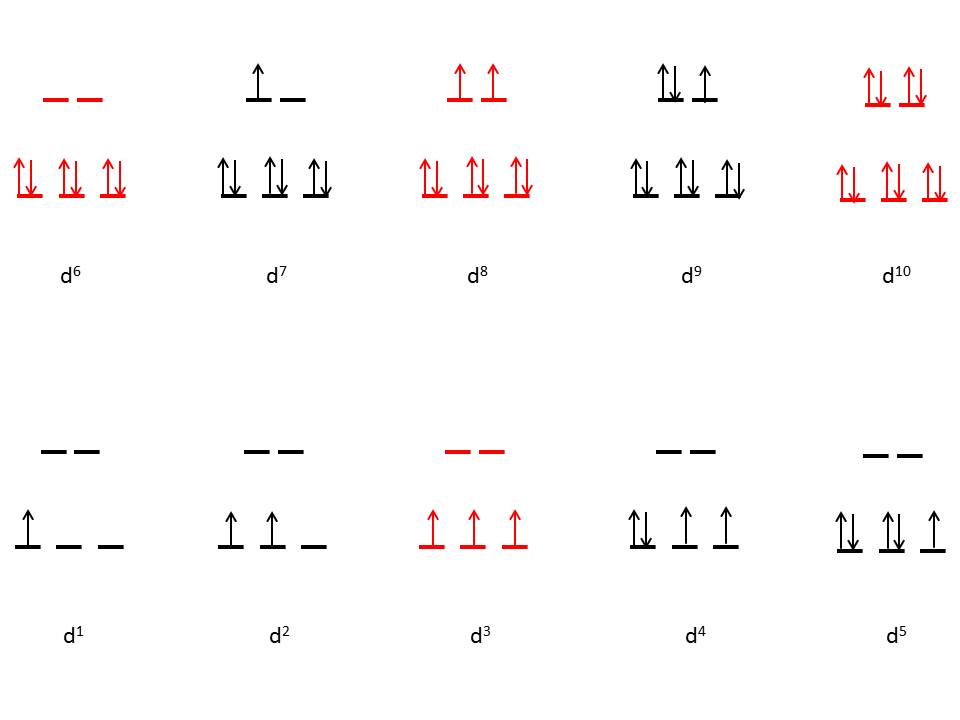
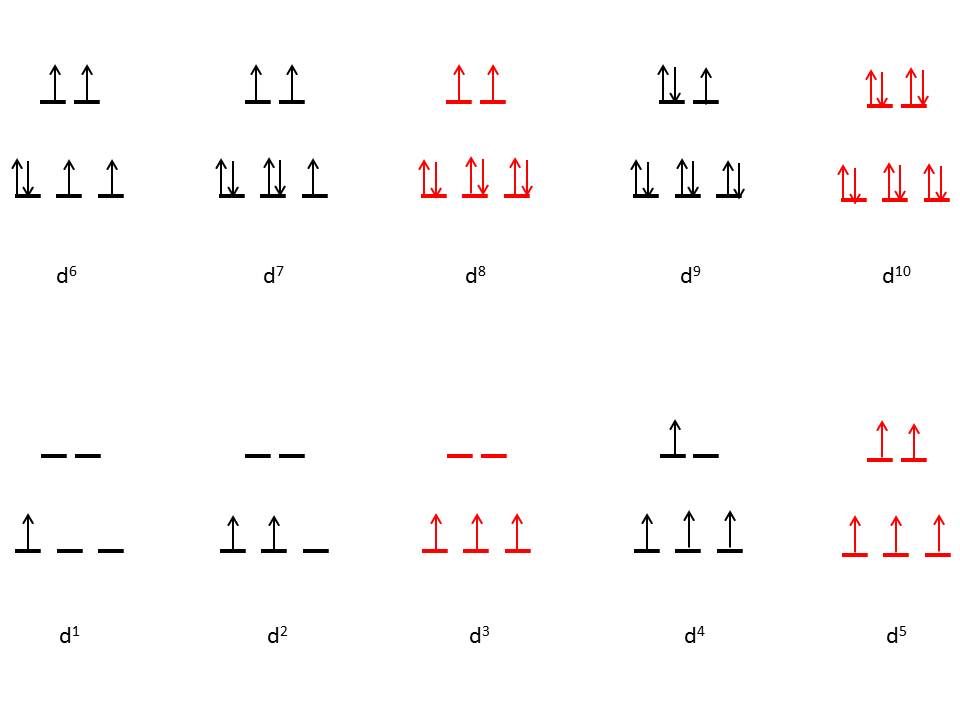
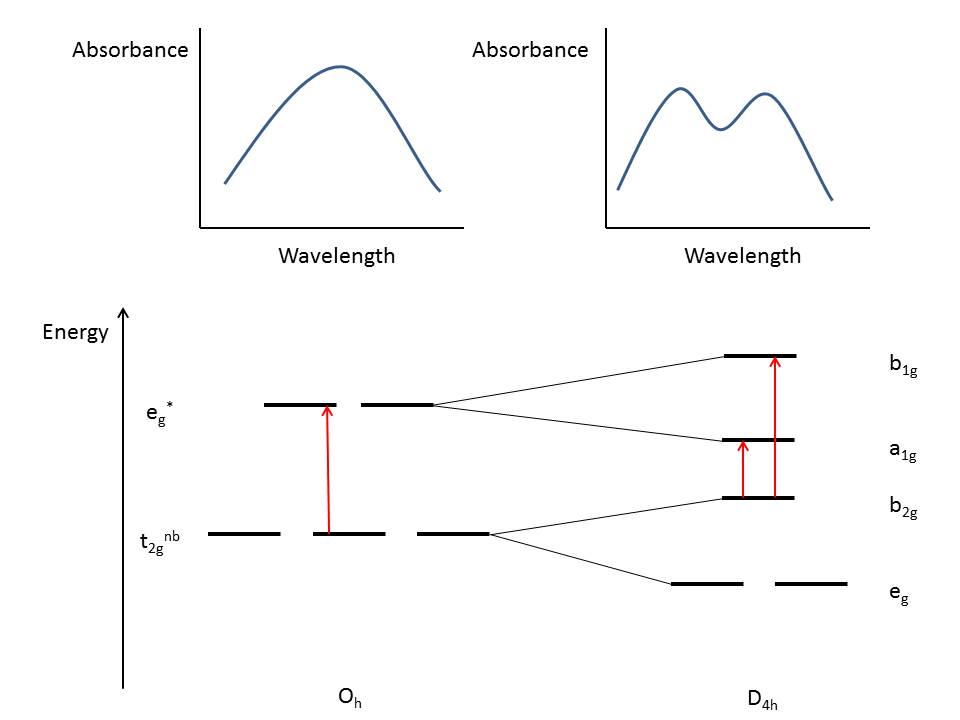
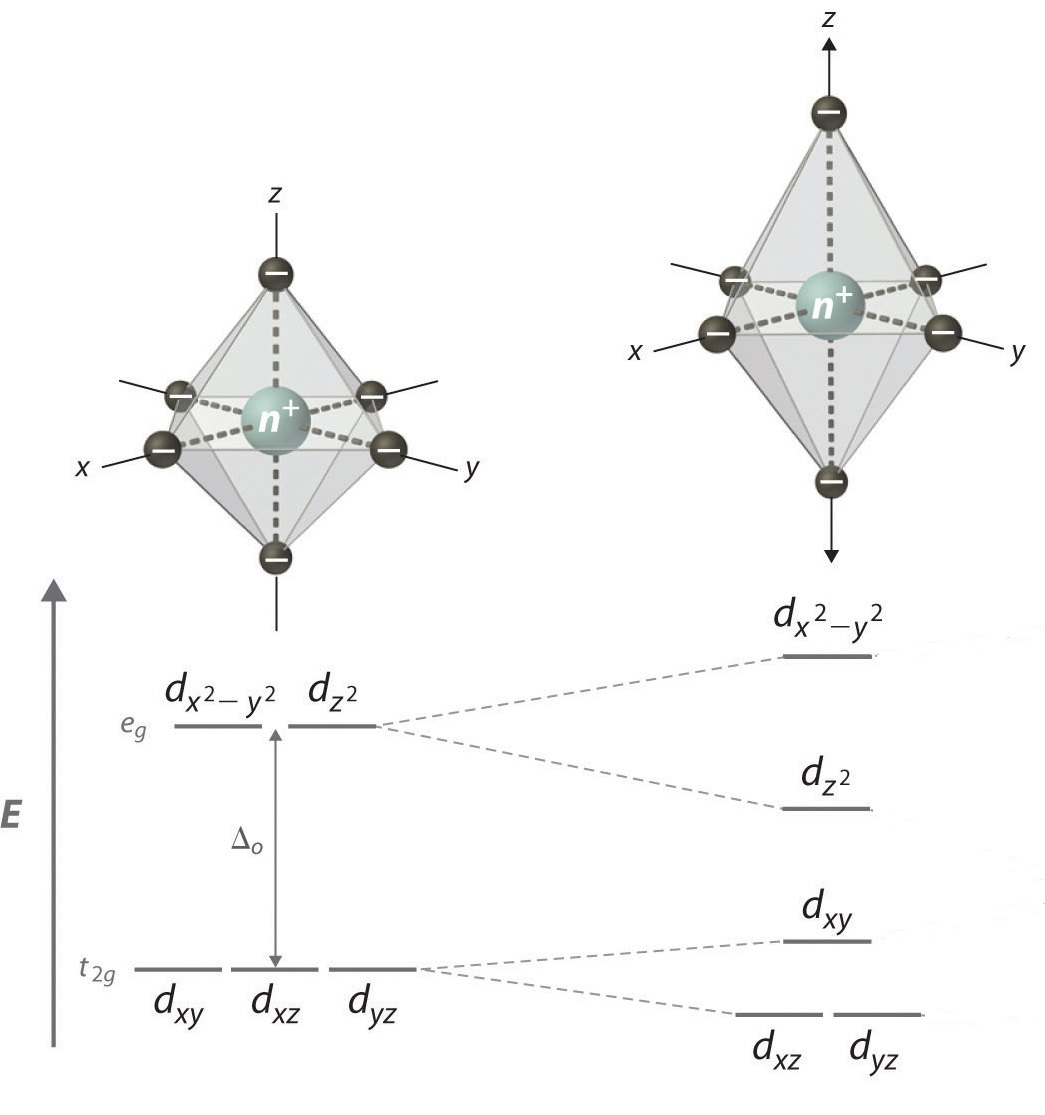
Change in geometry and arrangement of orbitals
 |
| Change in geometry and arrangement of orbitals in different Distortions |
Consequences of JT Distortion
Jahn Tellar Distortion leads to some amazing consesquences, some of which are given below:
1. Stability of Cu2+ ion
As given by the Irwing-William Series, the relative stabilities of complexes in (+2) oxidation states of different metals is as follows:
Ba2+ < Sr2+ < Ca2+
< Mg2+ < Mn2+ < Fe2+ < Co2+
< Ni2+ < Cu2+ > Zn2+
The
Cu2+ complexes are more stable than Zn
2+ complexes due to the Jahn-Tellar distortion observed in
Cu2+ complexes which is absent in the later.
2. Instability of Au2+ ion
Au
2+ disproportionate into Au
3+ and Au
1+ because in Au
2+ one electron is present in very high energy, which easily gets removed.
 |
| Orbital Splitting in ion Au2+ion (The 9th electron is in very high energy state) |
3. Instability of Chelating Complexes
[Cu(en)3]2+ is Not Stable. Why?
The above complex is a chelating complex of Cu2+ .This shows Jahn Tellar distortion. Cu-N bonds at axial positions try to elongate (due to JT Effect) but this elongation causes strain in the molecule. Hence the complex becomes unstable.
Practice Questions
- Why do d3 complexes not show Jahn-Teller distortions?
- Does the spin system (high spin v. low spin) of a molecule play a role in Jahn-Teller effects?
- What spectroscopic method would one utilize in order to observe Jahn-Teller distortions in a diamagnetic molecule?
- What spectroscopic method(s) would one utilize in order to observe Jahn-Teller distortions in a paramagnetic molecule?
- Why are Jahn-Teller effects most prevalent in inorganic (transition metal) compounds?





_fluoride.jpg?revision=1&size=bestfit&width=250&height=195)