The Jahn-Teller effect explains why certain 6 coordinated complexes undergo distortion to assume distorted octahedral (tetragonal) geometry. "This is a geometric distortion of a non-linear molecular system that reduces its symmetry and energy". This distortion is typically observed among octahedral complexes where the two axial bonds can be shorter or longer than those of the equatorial bonds. This effect can also be observed in tetrahedral compounds. This effect is dependent on the electronic state of the system.
Introduction
In 1937, Hermann Jahn and Edward Teller
postulated a theorem stating that "stability and degeneracy are not
possible simultaneously unless the molecule is a linear one," in regards
to its electronic state.This leads to a break in degeneracy which
stabilizes the molecule and by consequence, reduces its symmetry. Since
1937, the theorem has been revised which House-croft and Sharpe have
eloquently phrased as "any non-linear molecular system in a degenerate
electronic state will be unstable and will undergo distortion to form a
system of lower symmetry and lower energy, thereby removing the
degeneracy." This is most commonly observed with transition metal octahedral complexes, however, it can be observed in tetrahedral compounds as well.
For a given octahedral complex, the five d atomic orbitals are split into two degenerate sets when constructing a molecular orbital diagram. These are represented by the sets' symmetry labels: t2g (dxz, dyz, dxy) and eg (dz2 and dx2−y2). When a molecule possesses a degenerate electronic ground state, it will distort (Jahn-Teller effect) to remove the degeneracy and form a lower energy (and by consequence, lower symmetry) system. The octahedral complex will either elongate or compress the z ligand bonds as shown in Figure 1 below:
 |
| Figure 1: Jahn-Teller distortions for an octahedral complex. |
When an octahedral complex exhibits
elongation, the axial bonds are longer than the equatorial bonds. For a
compression, it is the reverse; the equatorial bonds are longer than the
axial bonds. Elongation and compression effects are dictated by the
amount of overlap between the metal and ligand orbitals. Thus, this
distortion varies greatly depending on the type of metal and ligands. In
general, the stronger the metal-ligand orbital interactions are, the
greater the chance for a Jahn-Teller effect to be observed.
Elongation (Z-Out distortion)
Elongation Jahn-Teller distortions occur
when the degeneracy is broken by the stabilization (lowering in energy)
of the d orbitals with a z component, while the orbitals without a z component are destabilized (higher in energy) as shown in Figure 2 below:
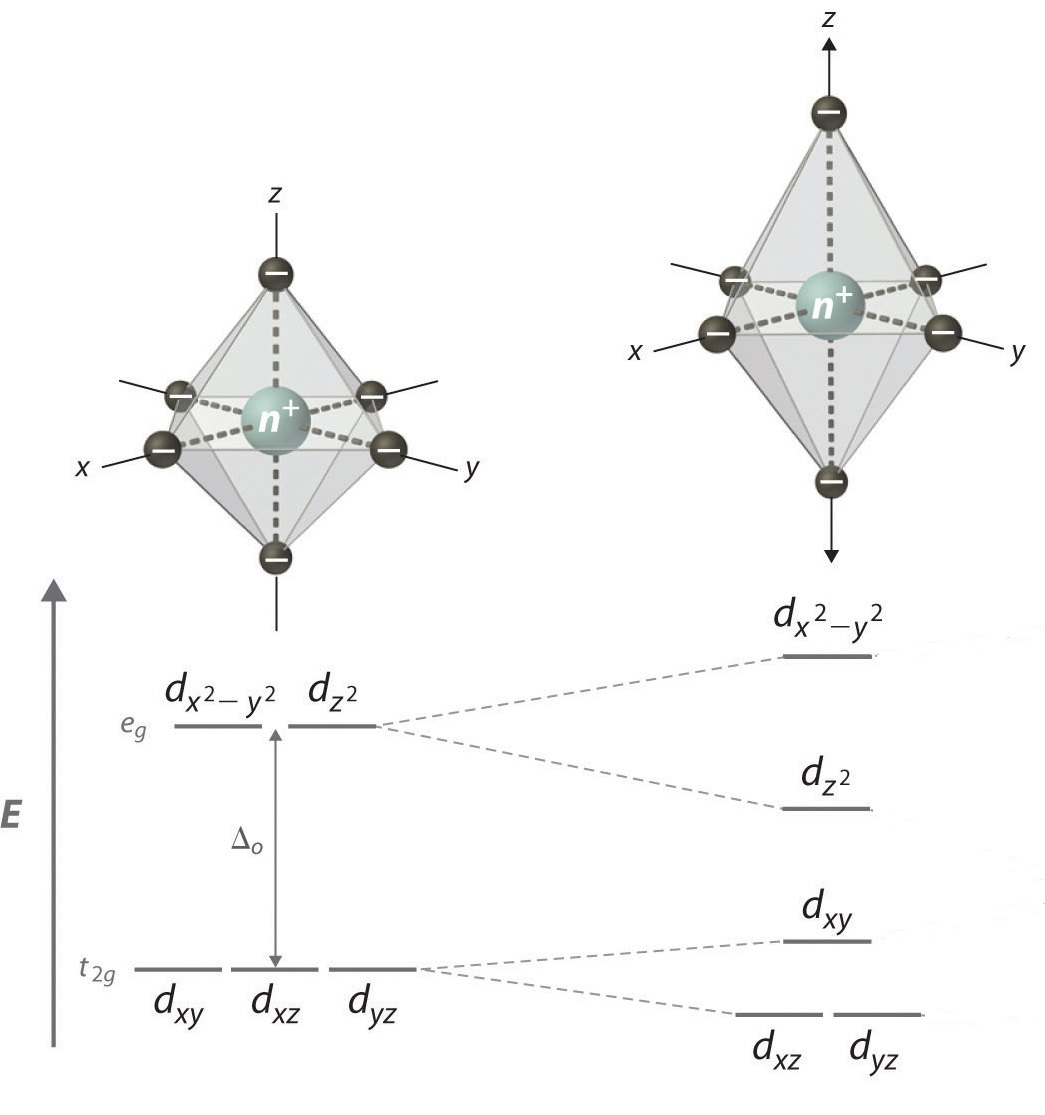 |
| Figure 2: Illustration of tetragonal distortion (elongation) for an octahedral complex. |
This is due to the dxy and dx2−y2 orbitals having greater overlap with the ligand orbitals, resulting in the orbitals being higher in energy. Since the dx2−y2 orbital
is antibonding, it is expected to increase in energy due to elongation.
The dxy orbital is still nonbonding, but is destabilized due to the
interactions.
Jahn-Teller elongations are well-documented for copper(II)
octahedral compounds. A classic example is that of copper(II) fluoride
as shown in Figure 3.
_fluoride.jpg?revision=1&size=bestfit&width=250&height=195) |
| Figure 3: Structure of octahedral copper(II) fluoride. |
Notice that the two axial bonds are both
elongated and the four shorter equatorial bonds are the same length as
each other. According the theorem, the orbital degeneracy is eliminated
by distortion, making the molecule more stable based on the model
presented in Figure 2.
Compression (Z-In distortion)
Compression Jahn-Teller distortions
occur when the degeneracy is broken by the stabilization (lowering in
energy) of the d orbitals without a z component, while the orbitals with a z component are destabilized (higher in energy) as shown in Figure 4 below:
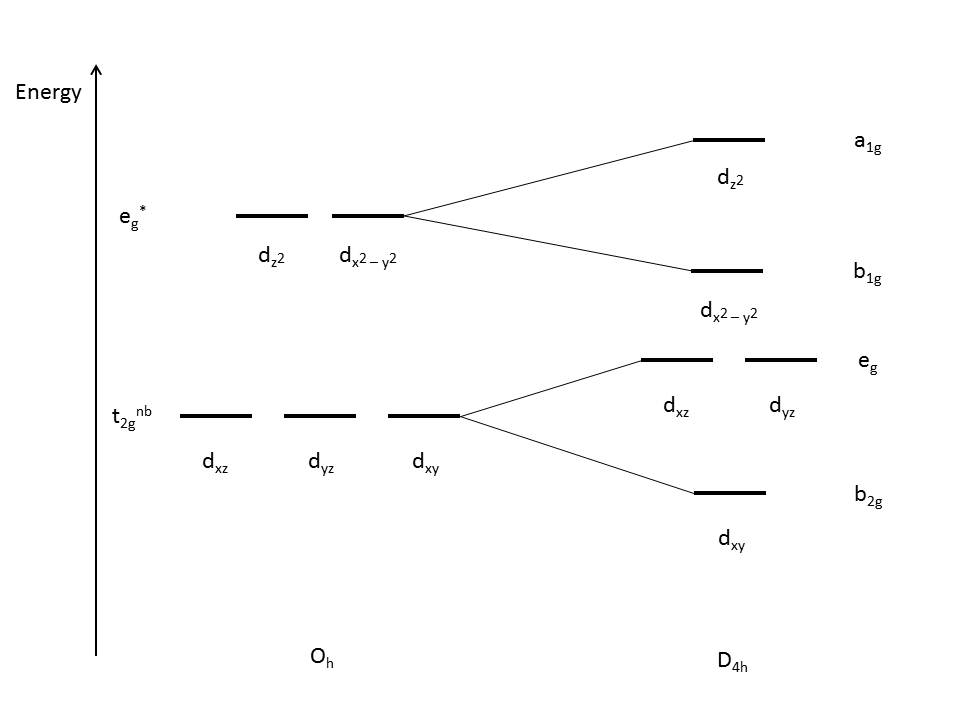 |
| Figure 4: Illustration of tetragonal distortion (compression) for an octahedral complex. |
This is due to the z-component d orbitals having greater overlap with the ligand orbitals, resulting in the orbitals being higher in energy. Since the dz2 orbital is anti-bonding, it is expected to increase in energy due to compression. The dxz and dyz orbitals are still non-bonding, but are destabilized due to the interactions.
Electronic Configurations and JT Distortions:
For Jahn-Teller effects to occur in transition metals there must be degeneracy in either the t2g or eg orbitals. The electronic states of octahedral complexes are classified as either low spin or high spin.
The spin of the system is dictated by the chemical environment. This
includes the characteristics of the metal center and the types of
ligands.
 |
| Distortions are more pronounced if the degeneracy occurs in an eg orbital |
Low Spin
Figure 5 (below) shows the various electronic configurations for low spin octahedral complexes, The Red Colored electronic configurations does not show any distortions whereas the Black Colored electronic configurations does: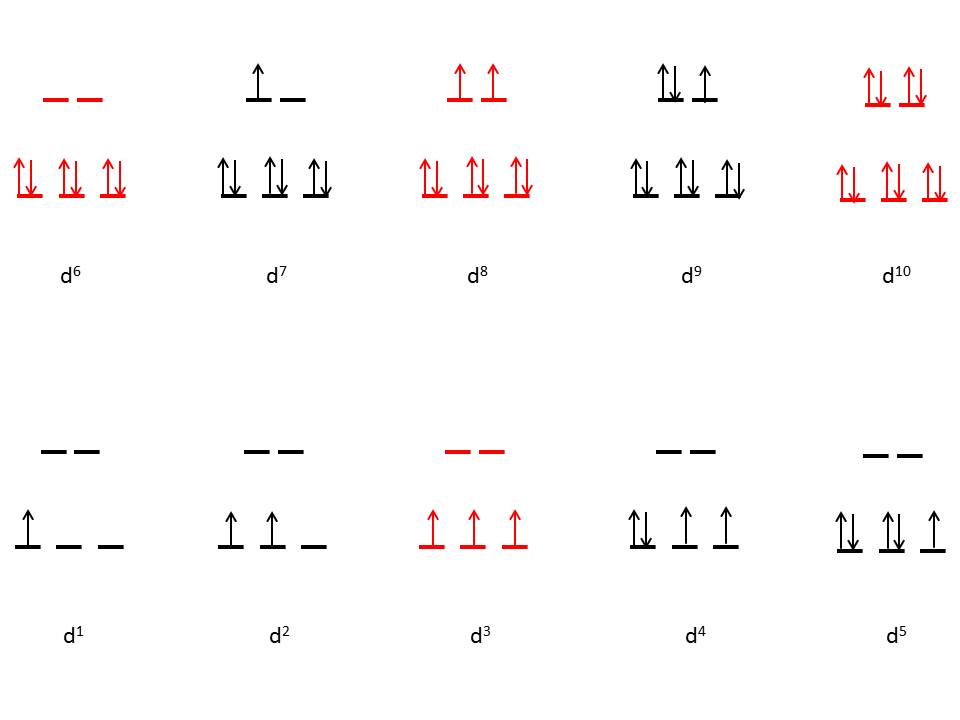 |
| Figure 5: Low spin octahedral coordination diagram (red indicates no degeneracies possible, thus no Jahn-Teller effects). |
The figure illustrates that low spin complexes with d3, d5, d8, and d10
electrons that do no exhibit Jahn-Teller distortions. These electronic
configurations correspond to a variety of transition metals. Many common
examples include Cr3+, Co3+, and Ni2+.
High Spin
Figure 6 (below) shows the various electronic configurations for high spin octahedral complexes, The Red Colored electronic configurations does not show any distortions whereas the Black Colored electronic configurations does: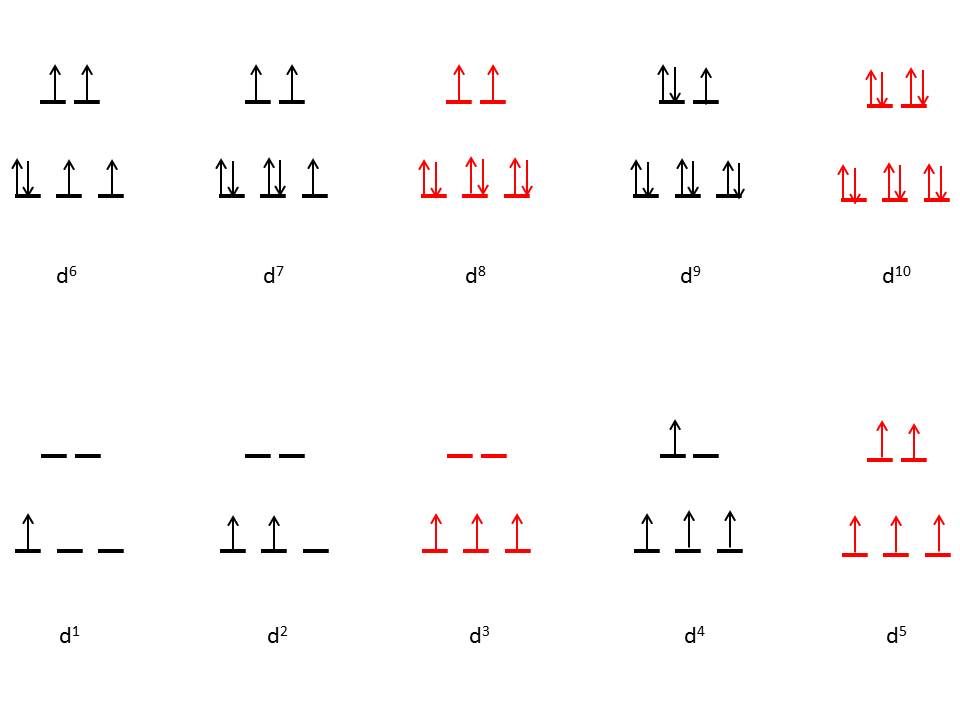 |
| Figure 6: High spin octahedral coordination diagram (red indicates no degeneracies possible, thus no Jahn-Teller effects) |
The figure illustrates that low spin complexes with d3, d5, d8, and d10 electrons
cannot have Jahn-Teller distortions. In general, degenerate electronic
states occupying the eg orbital set tend to show stronger Jahn-Teller
effects. This is primarily caused by the occupation of these high energy
orbitals. Since the system is more stable with a lower energy
configuration, the degeneracy of the eg set is broken, the symmetry is
reduced, and occupations at lower energy orbitals occur.
Spectroscopic Effects of JT Distortion
Jahn-Teller distortions can be observed
using a variety of spectroscopic techniques. In UV-VIS absorption
spectroscopy, distortion causes splitting of bands in the spectrum due
to a reduction in symmetry (Oh to D4h). Consider a
hypothetical molecule with octahedral symmetry showing a single
absorption band. If the molecule were to undergo Jahn-Teller distortion,
the number of bands would increase as shown in Figure 7 below:
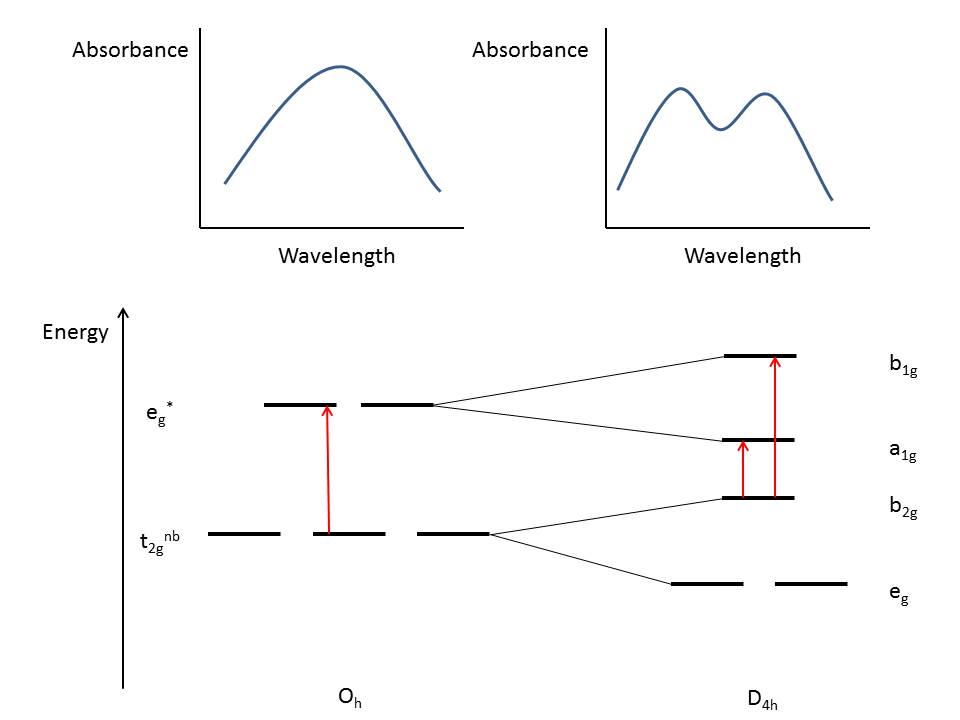 |
| Figure 7: Hypothetical absorption spectra of an octahedral molecule (left) and the same molecule with Jahn-Teller elongation (right). The red arrows indicate electronic transitions. |
A similar phenomenon can be seen with IR
and Raman vibrational spectroscopy. The number of vibrational modes for
a molecule can be calculated using the 3n - 6 rule (or 3n - 5 for
linear geometry) rule. If a molecule exhibits an Oh symmetry point group, it will have fewer bands than that of a Jahn-Teller distorted molecule with D4h symmetry. Thus, one could observe Jahn-Teller effects through either IR or Raman techniques. This effect can also be observed in EPR experiments as long as there is at least one unpaired electron.
| CuBr2 | 4 Br at 240 pm 2 Br at 318 pm |
|---|---|
| CuCl2 | 4 Cl at 230 pm 2 Cl at 295 pm |
| CuCl 2.2H 2O | 2 O at 193 pm 2 Cl at 228 pm 2 Cl at 295 pm |
| CsCuCl3 | 4 Cl at 230 pm 2 Cl at 265 pm |
| CuF2 | 4 F at 193 pm 2 F at 227 pm |
| CuSO4.4NH3.H2O | 4 N at 205 pm 1 O at 259 pm 1 O at 337 pm |
| K2CuF4 | 4 F at 191 pm 2 F at 237 pm |
| KCuAlF6 | 2 F at 188 pm 4 F at 220 pm |
| CrF2 | 4 F at 200 pm 2 F at 243 pm |
| KCrF3 | 4 F at 214 pm 2 F at 200 pm |
| MnF3 | 2 F at 209 pm 2 F at 191 pm 2 F at 179 pm |
The Jahn-Teller Theorem predicts that distortions should occur for any degenerate state, including degeneracy of the t2g level, however distortions in bond lengths are much more distinctive when the degenerate electrons are in the eg level.
Summary
We can summarize all the above contents, the type of distortion and the strength of distortion for different type of complexes in the below table:Change in geometry and arrangement of orbitals
 |
| Change in geometry and arrangement of orbitals in different Distortions |
Consequences of JT Distortion
Jahn Tellar Distortion leads to some amazing consesquences, some of which are given below:1. Stability of Cu2+ ion
As given by the Irwing-William Series, the relative stabilities of complexes in (+2) oxidation states of different metals is as follows:
Ba2+ < Sr2+ < Ca2+
< Mg2+ < Mn2+ < Fe2+ < Co2+
< Ni2+ < Cu2+ > Zn2+
The Cu2+ complexes are more stable than Zn2+ complexes due to the Jahn-Tellar distortion observed in Cu2+ complexes which is absent in the later.
2. Instability of Au2+ ion
Au2+ disproportionate into Au3+ and Au1+ because in Au2+ one electron is present in very high energy, which easily gets removed.
 |
| Orbital Splitting in ion Au2+ion (The 9th electron is in very high energy state) |
3. Instability of Chelating Complexes
[Cu(en)3]2+ is Not Stable. Why?
The above complex is a chelating complex of Cu2+ .This shows Jahn Tellar distortion. Cu-N bonds at axial positions try to elongate (due to JT Effect) but this elongation causes strain in the molecule. Hence the complex becomes unstable.
Practice Questions
- Why do d3 complexes not show Jahn-Teller distortions?
- Does the spin system (high spin v. low spin) of a molecule play a role in Jahn-Teller effects?
- What spectroscopic method would one utilize in order to observe Jahn-Teller distortions in a diamagnetic molecule?
- What spectroscopic method(s) would one utilize in order to observe Jahn-Teller distortions in a paramagnetic molecule?
- Why are Jahn-Teller effects most prevalent in inorganic (transition metal) compounds?
Answers
- Complexes with d3 electron configurations do not show Jahn-Teller distortions because there is no ground state degeneracy.
- Yes. Examining the d5 electron configuration, one finds that the high spin scenario contains all singly occupied d orbitals (no degeneracy). However, the low spin d5 electron configuration shows degeneracy, which then leads to possible Jahn-Teller effects.
- UV-VIS absorption spectroscopy is one of the most common techniques for observing these effects. In general, it is independent of magnetism (diamagnetic v. paramagnetic). Thus, one would see the effect in the spectrum of UV-VIS absorption analysis. Note that EPR requires at least one unpaired electron, and therefore not EPR active.
- Inorganic, specifically transition metal, complexes are most prevalent in showing Jahn-Teller distortions due to the availability of d orbitals. The most common geometry that the Jahn-Teller effect is observed is in octahedral complexes (see Figures 2, 4, 5 and 6 above) due to the splitting of d orbitals into two degenerate sets. Due to stabilization, the degeneracies are removed, making a lower symmetry and lower energy molecule.
- In addition to UV-VIS absorption, one can also employ EPR spectroscopy if a molecule possesses and unpaired electron.
References
- Jahn, H. A.; Teller, E. Proc. R. Soc. London A, 1937, 161, 220-235. DOI: 10.1098/rspa.1937.0142
- Housecroft, C.; Sharpe, A. G. Inorganic Chemistry. Prentice Hall, 3rd Ed., 2008, p. 644. ISBN: 978-0-13-175553-6
- Billy, C.; Haendler, H. A. J. Am. Chem. Soc., 1957, 79, 1049–1051. DOI: 10.1021/ja01562a011
- Chemistry Libretexts (https://chem.libretexts.org/)
- Wikipedia (https://en.wikipedia.org/wiki/Main_Page)
- Google (for images)






.png)


