In this post we will learn how to assign Nomenclature (closo, nido, arachno, hypo) to a given Borane and Also We'll Learn How to Calculate number of B-H-B bonds, B-B-B bonds in a given Borane through STYX code . According to Some pf the previous papers of CSIR, JAM and other Competitive Examinations, Questions related to this topic is frequently asked. I hope it'll help you and you'll be able to solve these questions easily after reading this post.
 |
| Source: google.com |
Structures and Bonding:
There are
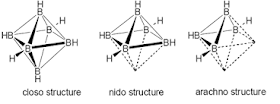
three important structures of boranes (closo-, nido- and arachno-) .In this
structures the boron atoms are occuping the corners of a polyhedron in which
the boron atoms can be bound together or not. These structures are so called
cage structures.
a) closo –
BnHn2- b)
nido – BnHn+4 c) arachno – BnHn+6
Examples:
a) B6H62-
is closo-type and the 6 B’s lie on the corners of a octahedron
b) B5H9 is nido-type
and the 5 B’s lie on the corners of a square pyramid where one corner is
removed
c) B4H10 is
arachno-type and the 4 B’s lie on the corners of an octaherdron where two
corners are removed
Boranes
have an electron-deficient, but they are coordinated by four atoms. This is the
reason why they are forming unusual bonds, because there are not enough
electrons to form the bonds. The dicription of the bonding in higher boranes
was formulated by William Lipscomb and is as follows:
3-center
2-electron B-H-B hydrogen bridges and 3-center 2-electron B-B-B bonds
Wade Rules
A second
method to determine the geometry of boranes are the Wade rules. The Wade rules
are a correlation between the number of electrons , the formula and the shape
of the molecule. To use this method, the total number of valence electrons that
are forming the bonds must be determined (n = Number of boron atoms).
Kenneth Wade (1932-2014) was a faculty at Durham University UK. In the early 1970’s he formulated the Wades rules which provided a major breakthrough in the qualitative understanding of the electron deficient multicentre bonding of boron hydrides and their shape based classification.
According to Wade his rule correlates skeletal structures of boranes, carboranes, hetero boranes and their anions (closo, nido, arachno, hypho) with the number of skeletal electron pairs they contain.
The rule states that clusters having n skeletal atoms (vertices) will adopt closo structures if it is held together by n+1 skeletal bonding electron pairs; nido if held together by n+2 skeletal electron pairs, arachno if held together by n+3 skeletal. electron pairs, hypho if held together by n+4 skeletal electron pairs and klado if held together by n+5 skeletal electron pairs.
For applying this rule one need to determine the number of skeletal electron pairs in a cluster. Each BH unit furnishes 2 skeletal bonding electrons, each B as such gives three skeletal electrons, each C-H unit of a carborane furnishes 3 skeletal bonding electrons and each additional H· furnishes 1 skeletal bonding electron. Ionic charges must be included in the electron count.
The rule states that clusters having n skeletal atoms (vertices) will adopt closo structures if it is held together by n+1 skeletal bonding electron pairs; nido if held together by n+2 skeletal electron pairs, arachno if held together by n+3 skeletal. electron pairs, hypho if held together by n+4 skeletal electron pairs and klado if held together by n+5 skeletal electron pairs.
For applying this rule one need to determine the number of skeletal electron pairs in a cluster. Each BH unit furnishes 2 skeletal bonding electrons, each B as such gives three skeletal electrons, each C-H unit of a carborane furnishes 3 skeletal bonding electrons and each additional H· furnishes 1 skeletal bonding electron. Ionic charges must be included in the electron count.
Extending this to borane clusters with other hetero-elements, one may replace C, Si, Ge and Sn of a cluster with a BH unit; N, P and As with a BH2 unit and S and Se with a BH3 unit for counting purpose.
Some Examples on Wade Rule:
Lipscomb's STYX codes:
The styx number
was introduced to aid in electron counting where
s = count of 3-center
B-H-B bonds;
t = count of 3-center B-B-B bonds;
t = count of 3-center B-B-B bonds;
y = count of 2-center
B-B bonds and
x = count of BH2 groups.
Lipscomb's methodology has
largely been superseded by a molecular orbital approach, although it still affords insights. The results of this have been summarized in a simple but powerful rule, PSEPT (Polyhedral Skeletal Electron Pair Theory), often known as Wade's rules, that can be used to predict the cluster type, closo-, nido-, etc. The power of this rule is its ease of use and general applicability to many different cluster types other than boranes."
Here is a method to Calculate STYX code for different boranes.
NOTE: For Compounds having B6 or more and Compounds with B-H difference of 4 is directly taken as S values,
for example. In case of B6H10 diff is 4 so S =4 we will not take 2, _, _, 2. But we will take 4, _, _,0 as S and X values
for example. In case of B6H10 diff is 4 so S =4 we will not take 2, _, _, 2. But we will take 4, _, _,0 as S and X values
- You can also calculate STYX code directly just by entering the type of boron you want to calculate for by going through this link: STYX Calculator





Thankyou..
ReplyDeleteYou're Welcome :)
DeleteCould you please send me notes on computational chemistry and nanochemistry.......
ReplyDeleteThanks for ur other notes.... It help me alot
Sir can You give the trick for how we calculate different metal- metal bond in organometallic compound? Eg.Co2Fe2(CO)11(mu4-PPh)2]
ReplyDeleteCan you give it in pdf formate plz....
ReplyDeletesir give me the trick to solve metal metal.bonds and bridging ligands and boran structures
ReplyDeletesir give me the trick to solve metal metal.bonds and bridging ligands and boran structures
ReplyDeleteGreat content.. helpful in understanding STYX codes.
ReplyDelete